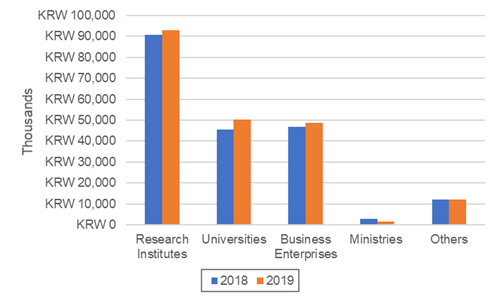
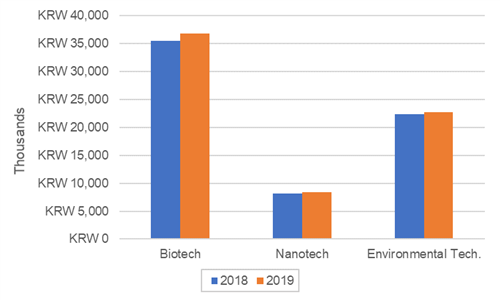
UK
The UK's licensed gene and cell therapy manufacturing capacity continues to grow. A survey conducted by the Cell and Gene Therapy Catapult in October and November encompassed 25 out of 26 facilities (see table). Of these, 11 are commercially owned with the remainder in the academic and the public sector.
In total, manufacturing footprint (cleanroom space) increased 47.5% between November 2019 and November 2020 to reach 126,541 ft2 (11,756 m2) with a total of 159 cleanrooms. In-house QC space rose 9.9% to 98,834 ft2 (9,182 m2). New or expanded space was added by Oxford BioMedica, Cobra Biologics, and the Cell and Gene Therapy Catapult. During the coming 12 months, 700 m2 of additional space should be licensed.
Average booking capacity was 86% for the period, up 14%. Full-time staffing for manufacturing, test and release rose 15.0% to 1,310.
# of Facilities | Number of Cleanrooms | Total Cleanroom Space (m2) | Chg. in Cleanroom Space | In-hose QC Space (m2) | Full-time Employees | Avg. Booked Capacity | |
|---|---|---|---|---|---|---|---|
Cell Therapy | 11 | 56 | 1,517 | 8.0% | 483 | 154 | 86% |
Gene Therapy | 8 | 57 | 6,694 | 72.1% | 7,849 | 603 | 95% |
Multifunctional | 7 | NA | 3,545 | 32.5% | 850 | 553 | 75% |
The top capabilities at UK gene therapy manufacturing sites, out of 8 categories of processes provided, were suspension and adherence (9 sites each), and lentivirus and AAV (8 each).
Among both cell therapy and multifunctional sites, the greater number of sites, out of 8 process categories provided, were allogenic and/or autologous processes (15 sites each), followed by tissue-specific stem cells (14) and immune cells (14).
Source: Cell and Gene Therapy Catapult (December 1)